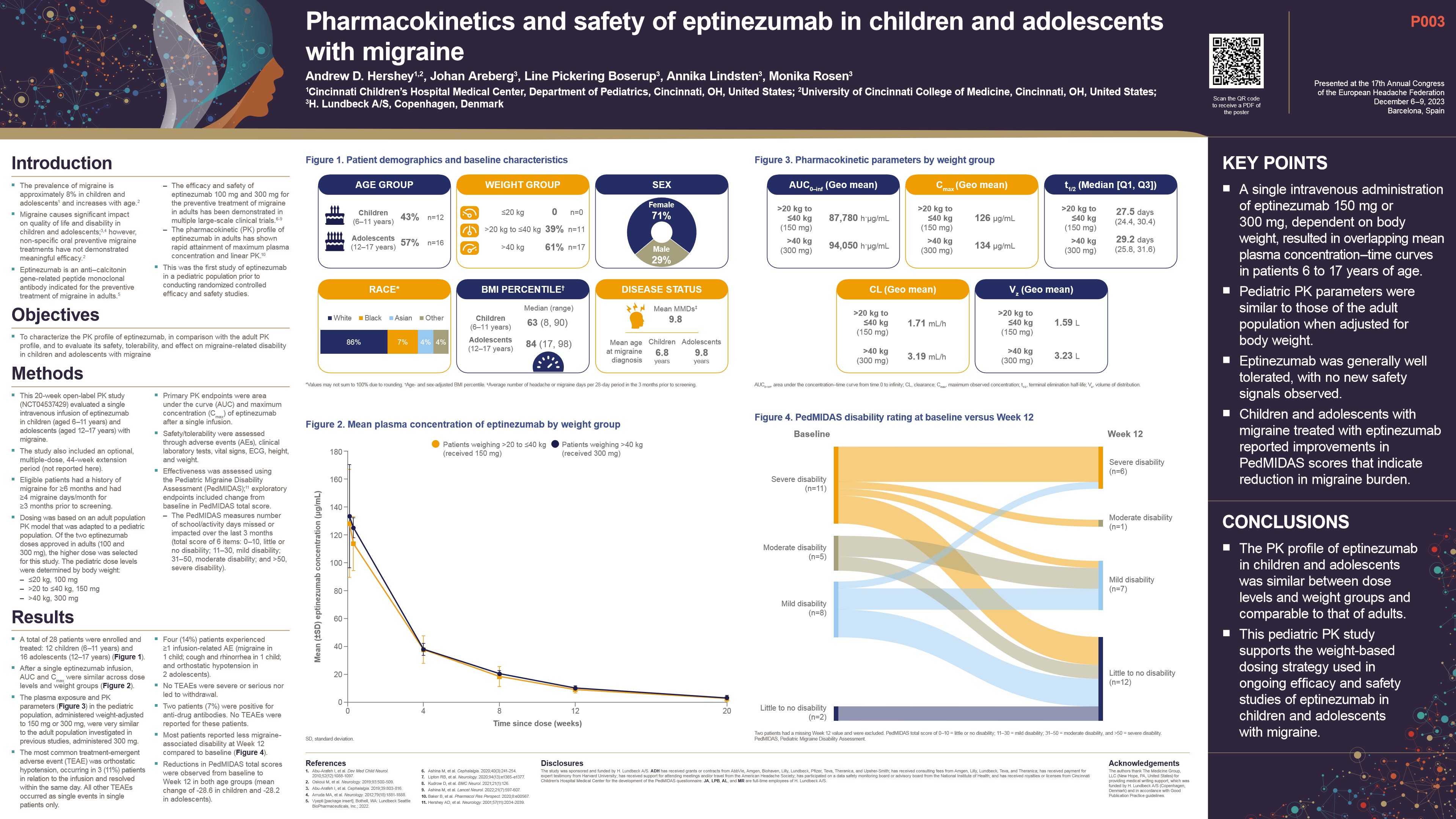
Weight based dosing with eptinezumab was generally well tolerated by children and adolescents, according to results of an open label pharmacokinetic (PK) study which used a population PK (PopPK) model adapted to a paediatric population.
The 20-week open label study (NCT04537429) evaluated a single intravenous infusion of eptinezumab in children and adolescents (aged 6-17 years) with migraine. Eligible patients had a history of ≥4 migraine days/month for ≥3 months prior to screening, and dose levels by body weight were: ≤20 kg, 100 mg; >20 to ≤40 kg, 150 mg; >40 kg, 300 mg.
A total of 28 patients were enrolled, mainly female (20/28) and white (24/28), with a mean age of 12.6 years and body weight ranging 21-79 kg. After a single eptinezumab infusion, AUC and Cmax were similar across dose levels and weight groups and overlapped with adult-based prediction models. Four patients (14.3%) experienced ≥1 infusion-related AE. These were migraine in one child, cough and rhinorrhea in one child, and orthostatic hypotension in two adolescents. No treatment-emergent AEs were severe or serious or led to withdrawal. The mean PedMIDAS score decreased from 52.7 (severe) at baseline to 25.1 (mild) at week 12.
Reference
Hershey AD, Areberg J, Boserup LP et al. Pharmacokinetics and safety of eptinezumab in children and adolescents with migraine. Presented at the 17th European Headache Congress, Barcelona, Spain, 6-9 December 2023; PoS 1, e-poster 01, PO03